CLINICAL DATA:
A 47-year-old man presented with worsening bilateral orbital swelling, parotid swelling and submandibular swelling for several months.
ESR 90mm/hr
Creatinine 150umol/l
Elevated serum uric acid
IMAGING INVESTIGATIONS:



- Bilateral swelling of the lacrimal glands. Homogenous enhancement of the involved glands with no liquefaction/calcification.



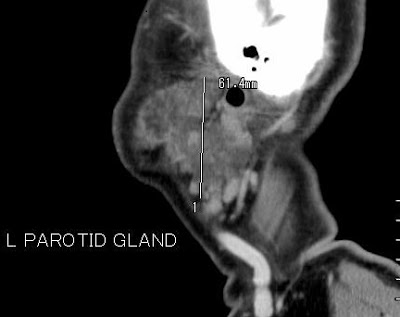
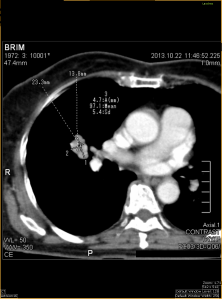
- Bilateral swelling of parotid glands. Heterogenous enhancement predominantly in the periphery of the glands. No liquefaction/calcification.
- No enlarged neck node.
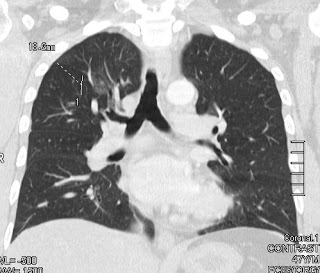

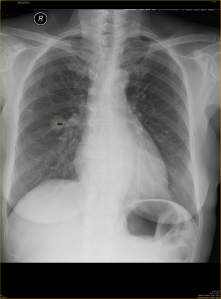
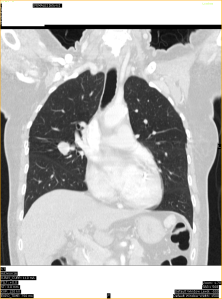
- Focal ground glass opacity in the right upper lobe.

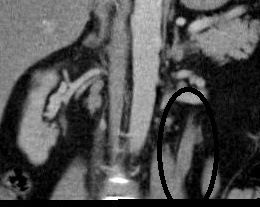

- Ureteric wall thickening of the visualized upper left ureter.
- Mild left hydronephrosis.
- characterized by fibro-inflammatory lesions rich in IgG4-positive plasma cells and, often but not always, elevated serum IgG4 concentrations.
- the pathogenesis of IgG4-related disease is poorly understood, but there are findings consistent with both an autoimmune and an allergic disorder.
- was recognized as a systemic disease in 2003, when extrapancreatic manifestations were identified in patients with autoimmune pancreatitis. The pancreas is the most commonly affected organ in IgG4-related disease.
- affecting virtually every organ system and has been identified in the biliary tree, salivary and lacrimal glands, periorbital tissues, lungs, lymph nodes, thyroid gland, kidneys, prostate gland, testicles, breasts, skin, meninges, retroperitonrum, mesentery, and pituitary gland (1-3).
- Extrapancreatic lesions may sometimes develop before or up to 15 years after pancreatitis.
- imaging plays an important role in demonstrating infiltration and enlargement of involved organs.
- Immunohistochemical staining for IgG4 is required. The presence of IgG4-positive plasma cells is required for diagnosis of IgG4-related disease, but some IgG4-positive plasma cells can also be detected in other inflammatory diseases. However, it has been suggested that dense infiltrates of IgG4-plasma cells (>10–50 per high-power microscopic field, depending on the affected organ) are highly specific for IgG4-related disease (3). A ratio of IgG4-positive to IgG-positive plasma cells higher than 40% is also helpful in distinguishing between IgG4-related disease and non–IgG4-related inflammatory conditions (3-5).
- Elevated serum IgG4 concentration is common in but not specific for IgG4-related disease (6).
- Serum IgG4 concentration may be normal in 20%–40% of cases of biopsy-proved IgG4-related disease (3, 7,8). Thus, neither an increase in serum IgG4 concentration nor an elevated number of IgG4-positive plasma cells in tissue is specific for IgG4-related disease (18).
- Response to steroid therapy is excellent in most patients, although some patients are refractory to such therapy (7).
- Nearly 40% of patients with IgG4-related pancreatitis also have salivary and/or lacrimal gland involvement, which is characterized by bilateral and painless swelling of the glands (9).This manifestation may precede or accompany pancreatitis, but it may also occur in isolation.
- The pancreas is the most common site affected, followed by the biliary tree and kidneys, but virtually any site can be involved, including the gallbladder, lymph nodes, retroperitoneum, mesentery, lungs, breast, prostate, skin, and a variety of areas in the head and neck.
- Recent reports suggest that a substantial proportion of idiopathic orbital inflammations—also called orbital pseudotumors—are associated with IgG4-related disease(10).
- Renal involvement may be present in up to 35% of patients with autoimmune pancreatitis (11,12). The presence of renal lesions in patients with pancreatic disease has been used to help differentiate autoimmune pancreatitis from pancreatic cancer, since their presence strongly suggests the former condition. Five patterns of disease have been described:
- bilateral round or wedge-shaped peripheral cortical lesions (the most common)
- diffuse patchy involvement
- a rim of soft tissue around the kidney,
- bilateral nodules in the renal sinuses, and diffuse wall thickening of the renal pelvis (11).
- Patients with IgG4-related nephritis have no hematuria and usually improve after undergoing corticosteroid therapy.
- Four major types of IgG4-related pulmonary disease have been defined:
- (a) a type characterized by solid nodular or mass like lesions,
- (b) a type characterized by round ground-glass opacities,
- (c) alveolar interstitial disease, and
- (d) bronchovascular disease (13).
- When IgG4-related lung disease appears as a round ground-glass opacity at CT, imaging findings may suggest bronchoalveolar carcinoma.
- Visceral or parietal pleural thickening has been reported, sometimes involving the subpleural lung parenchyma